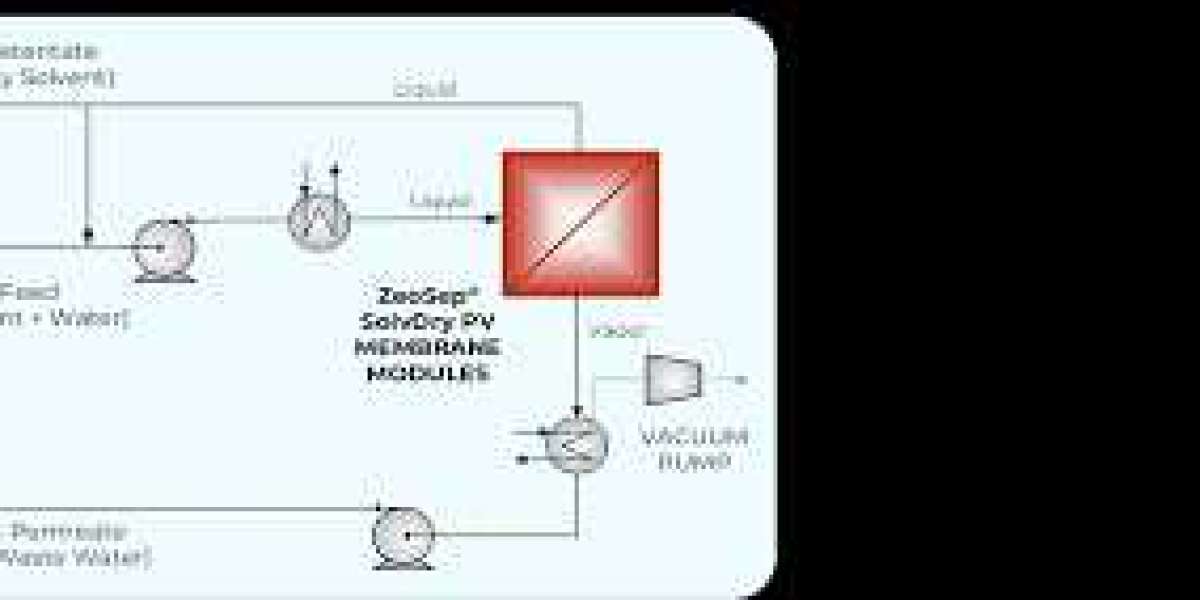
Chemical industries in India and the USA are under mounting pressure to adopt cleaner, resource-efficient processes. Separation of solvents and water is a key unit operation in countless manufacturing workflows, from pharmaceuticals to petrochemicals. Pervaporation stands out as one of the most advanced membrane-based approaches, replacing energy-heavy distillation with methods that are selective, precise, and aligned with environmental imperatives.
The Shift from Conventional Methods
Why Old Techniques Fall Short
- Evaporation and distillation have dominated the chemical separation landscape for decades. However, these legacy methods consume substantial energy and often yield below-optimal purity levels, especially when challenged by azeotropes or heat-sensitive compounds. Pervaporation uses high-performance membranes to separate liquids based on molecular size and affinity, greatly reducing overall energy requirements while delivering remarkable product quality.
Efficiency and Cost Reduction
- Traditional solvent recovery and drying systems face high operational costs due to heating demands. Pervaporation operates effectively at lower temperatures, helping manufacturers decrease utility bills. This energy efficiency is particularly valuable in regions like India, where cost pressure can influence the viability of advanced processes.
What Sets Pervaporation Apart?
Precision with Advanced Membranes
Pervaporation uses selective membranes engineered to let specific chemicals through while holding back others. This precise targeting is valuable for separating close-boiling or azeotropic mixtures—a task where distillation can falter. As the chemical mixture passes through the membrane, components with greater affinity cross over, yielding high-purity outputs suitable for stringent downstream applications.
Sustainability Advantages
Lower carbon emissions due to reduced energy input.
Minimal need for hazardous entrainers or additives, making manufacturing safer.
Smaller equipment footprints, scaling readily for diverse plant requirements.
Effective for solvent dehydration, removal of organics from water, and specialty separations required in fine chemical and pharmaceutical production.
Emerging Trends in Pervaporation
Technological Progress
- Modern pervaporation membranes employ materials like zeolites, polymers, or hybrid composites. These advances have led to greater selectivity and flux, boosting productivity without sacrificing stability. Ongoing innovations focus on resisting fouling and accommodating a wider array of solvents, so integration is feasible in everything from batch reactors to continuous-flow systems.
Industrial Applications Expanding
- Sectors benefiting from pervaporation extend beyond laboratory-scale use. Pharmaceutical manufacturing, ethanol production, solvent recycling facilities, and specialty chemicals are deploying pervaporation to address issues such as solvent drying and recovery of valuable intermediates. Indian and American manufacturers are converging on this approach due to both regulatory drivers and market incentives.
Implementation: Key Points for Decision Makers
Understand the nature of the feed mixture: precise membrane choice is crucial.
Evaluate integration opportunities with existing systems.
Anticipate reduced environmental impact and compliance with emerging regulations.
Plan for scalability, from pilot plants to full-capacity installations.
Conclusion
Adopting pervaporation allows chemical manufacturers to meet efficiency, sustainability, and quality benchmarks demanded by international customers and environmental authorities. As regulatory and financial pressures intensify across India and the USA, pervaporation isn’t just an alternative—it is fast becoming a best practice for advanced liquid separations. Solutions from I3 Nanotec empower organizations to realize these gains, maximizing both operational reliability and eco-performance.